Chemical Engineering - Chemical Engineering Thermodynamics
Exercise : Chemical Engineering Thermodynamics - Section 6
- Chemical Engineering Thermodynamics - Section 1
- Chemical Engineering Thermodynamics - Section 2
- Chemical Engineering Thermodynamics - Section 3
- Chemical Engineering Thermodynamics - Section 4
- Chemical Engineering Thermodynamics - Section 5
- Chemical Engineering Thermodynamics - Section 6
- Chemical Engineering Thermodynamics - Section 7
- Chemical Engineering Thermodynamics - Section 8
- Chemical Engineering Thermodynamics - Section 9
- Chemical Engineering Thermodynamics - Section 10
- Chemical Engineering Thermodynamics - Section 11
31.
Gibbs free energy at constant pressure and temperature under equilibrium conditions is
32.
With increase in pressure (above atmospheric pressure), the Cp of a gas
33.
A change in state involving a decrease in entropy can be spontaneous, only if
34.
Joule-Thomson co-efficient which is defined as, 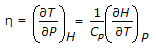 , changes sign at a temperature known as inversion temperature. The value of Joule-Thomson co-efficient at inversion temperature is
, changes sign at a temperature known as inversion temperature. The value of Joule-Thomson co-efficient at inversion temperature is
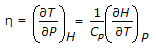 , changes sign at a temperature known as inversion temperature. The value of Joule-Thomson co-efficient at inversion temperature is
, changes sign at a temperature known as inversion temperature. The value of Joule-Thomson co-efficient at inversion temperature is35.
__________ calorimeter is normally used for measuring the dryness fraction of steam, when it is very low.
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers