Chemical Engineering - Chemical Engineering Thermodynamics
Exercise : Chemical Engineering Thermodynamics - Section 3
- Chemical Engineering Thermodynamics - Section 1
- Chemical Engineering Thermodynamics - Section 2
- Chemical Engineering Thermodynamics - Section 3
- Chemical Engineering Thermodynamics - Section 4
- Chemical Engineering Thermodynamics - Section 5
- Chemical Engineering Thermodynamics - Section 6
- Chemical Engineering Thermodynamics - Section 7
- Chemical Engineering Thermodynamics - Section 8
- Chemical Engineering Thermodynamics - Section 9
- Chemical Engineering Thermodynamics - Section 10
- Chemical Engineering Thermodynamics - Section 11
36.
Fugacity is most helpful in
37.
The energy of activation of exothermic reaction is
38.
Pick out the wrong statement.
39.
In the reaction; N2 + O2  2NO, increasing the pressure will result in
2NO, increasing the pressure will result in
 2NO, increasing the pressure will result in
2NO, increasing the pressure will result in40.
A cyclic engine exchanges heat with two reservoirs maintained at 100 and 300°C respectively. The maximum work (in J) that can be obtained from 1000 J of heat extracted from the hot reservoir is
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers
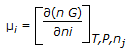 , where, n, ni and nj respectively denote the total number of moles, moles of ith species and all mole numbers except ith species. 'G' is Gibbs molar free energy.
, where, n, ni and nj respectively denote the total number of moles, moles of ith species and all mole numbers except ith species. 'G' is Gibbs molar free energy.