Online Chemical Engineering Test - Thermodynamics Test 1
Instruction:
- This is a FREE online test. Beware of scammers who ask for money to attend this test.
- Total number of questions: 20.
- Time allotted: 30 minutes.
- Each question carries 1 mark; there are no negative marks.
- DO NOT refresh the page.
- All the best!
Marks : 2/20
Total number of questions
20
Number of answered questions
0
Number of unanswered questions
20
Test Review : View answers and explanation for this test.
1.
Heat pump
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
2.
An isolated system can exchange __________ with its surroundings.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
3.
Pick out the wrong statement.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
4.
Equation which relates pressure, volume and temperature of a gas is called the
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
5.
The entropy change in a reversible isothermal process, when an ideal gas expands to four times its initial volume is
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
6.
Free energy, fugacity and activity co-efficient are all affected by change in the temperature. The fugacity co-efficient of a gas at constant pressure ____with the increase of reduced temperature.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
7.
For an irreversible process involving only pressure-volume work
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
8.
For a given substance at a specified temperature, activity is __________ to fugacity.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
9.
If we increase the pressure on a substance (which is at its triple point), then the triple point
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
10.
Pick out the wrong statement.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
11.
Joule-Thomson co-efficient which is defined as, 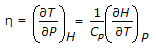 , changes sign at a temperature known as inversion temperature. The value of Joule-Thomson co-efficient at inversion temperature is
, changes sign at a temperature known as inversion temperature. The value of Joule-Thomson co-efficient at inversion temperature is
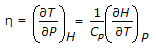 , changes sign at a temperature known as inversion temperature. The value of Joule-Thomson co-efficient at inversion temperature is
, changes sign at a temperature known as inversion temperature. The value of Joule-Thomson co-efficient at inversion temperature isYour Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
12.
In any spontaneous process, the __________ free energy decreases.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
13.
Air-refrigeration cycle
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
14.
The temperature at the eutectic point of the system is the __________ temperature that can be attained in the system.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
15.
Melting of ice is an example of an __________ process.
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
16.
Steam undergoes isentropic expansion in a turbine from 5000 kPa and 400°C (entropy = 6.65 kJ/kg K) to 150 kPa) (entropy of saturated liquid = 1.4336 kJ/kg . K, entropy of saturated vapour = 7.2234 kJ/kg. K) The exit condition of steam is
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
17.
Gibbs free energy (F) is defined as
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
18.
Fugacity and pressure are numerically not equal for the gases
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
19.
For an ideal liquid solution, which of the following is unity ?
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
20.
For any system, what is the minimum number of degrees of freedom?
Your Answer: Option
(Not Answered)
Correct Answer: Option
Discuss about this problem : Discuss in Forum
Learn more problems on : Chemical Engineering Thermodynamics
*** END OF THE TEST ***
Time Left: 00:29:56
Post your test result / feedback here:
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers