Chemical Engineering - Chemical Reaction Engineering
Exercise : Chemical Reaction Engineering - Section 4
- Chemical Reaction Engineering - Section 1
- Chemical Reaction Engineering - Section 2
- Chemical Reaction Engineering - Section 3
- Chemical Reaction Engineering - Section 4
- Chemical Reaction Engineering - Section 5
- Chemical Reaction Engineering - Section 6
- Chemical Reaction Engineering - Section 7
- Chemical Reaction Engineering - Section 8
- Chemical Reaction Engineering - Section 9
- Chemical Reaction Engineering - Section 10
16.
The reaction A  B is conducted in an adiabatic plug flow reactor (PFR). Pure A at a concentration of 2 kmol/m3 is fed to the reactor at the rate of 0.01 m3 /s and at a temperature of 500 K. If the exit conversion is 20%, then the exit temperature (in k)is (Data: Heat of reaction at 298 K = - 50000 kJ/ kmole of A reacted Heat capacities CPA = CPB = 100kJ/kmole. K (may be assumed to be independent of temperature))
B is conducted in an adiabatic plug flow reactor (PFR). Pure A at a concentration of 2 kmol/m3 is fed to the reactor at the rate of 0.01 m3 /s and at a temperature of 500 K. If the exit conversion is 20%, then the exit temperature (in k)is (Data: Heat of reaction at 298 K = - 50000 kJ/ kmole of A reacted Heat capacities CPA = CPB = 100kJ/kmole. K (may be assumed to be independent of temperature))
 B is conducted in an adiabatic plug flow reactor (PFR). Pure A at a concentration of 2 kmol/m3 is fed to the reactor at the rate of 0.01 m3 /s and at a temperature of 500 K. If the exit conversion is 20%, then the exit temperature (in k)is (Data: Heat of reaction at 298 K = - 50000 kJ/ kmole of A reacted Heat capacities CPA = CPB = 100kJ/kmole. K (may be assumed to be independent of temperature))
B is conducted in an adiabatic plug flow reactor (PFR). Pure A at a concentration of 2 kmol/m3 is fed to the reactor at the rate of 0.01 m3 /s and at a temperature of 500 K. If the exit conversion is 20%, then the exit temperature (in k)is (Data: Heat of reaction at 298 K = - 50000 kJ/ kmole of A reacted Heat capacities CPA = CPB = 100kJ/kmole. K (may be assumed to be independent of temperature))17.
Which of the following is not a theory of homogeneous reaction?
18.
A first order homogeneous reaction of the type X  Y
Y  Z (consecutive reaction) is carried out in a CSTR. Which of the following curves respectively show the variation of the concentration of X, Y and Z with time?
Z (consecutive reaction) is carried out in a CSTR. Which of the following curves respectively show the variation of the concentration of X, Y and Z with time?
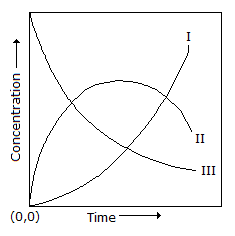
 Y
Y  Z (consecutive reaction) is carried out in a CSTR. Which of the following curves respectively show the variation of the concentration of X, Y and Z with time?
Z (consecutive reaction) is carried out in a CSTR. Which of the following curves respectively show the variation of the concentration of X, Y and Z with time? 
19.
In a semi-batch reactor,
20.
Most important characteristics of gas-liquid reactors are the
Quick links
Quantitative Aptitude
Verbal (English)
Reasoning
Programming
Interview
Placement Papers